Green hydrogen is rapidly emerging as an indispensable energy carrier in the global transition to a sustainable future. As a clean, carbon-neutral fuel, it holds the key to decarbonizing our economy's most challenging sectors, from heavy industry and long-haul transport to seasonal energy storage.
For landowners, investors, and governments, understanding how green hydrogen is produced is the first step toward capitalizing on the immense opportunities it presents. To appreciate its significance, we first need to understand the "hydrogen color spectrum," an industry shorthand for classifying hydrogen based on how it's made.
Green Hydrogen: The gold standard. It's produced through water electrolysis powered exclusively by renewable energy like solar and wind. The process is entirely carbon-free.
Grey Hydrogen: The incumbent. This is how over 95% of hydrogen is made today, using natural gas in a carbon-intensive process called Steam Methane Reforming (SMR).
Blue Hydrogen: A transitional step. This is grey hydrogen, but with a large portion of the carbon emissions captured and stored underground. It's a "low-carbon," but not zero-carbon, solution.
While these color codes are a useful starting point, the industry is moving toward a more precise metric: carbon intensity, which measures the lifecycle emissions of production. This ensures that when we talk about "green," we are referring to a genuinely clean process from start to finish. This guide provides a comprehensive overview of how true green hydrogen is made.
The Heart of Production: How Water Electrolysis Works
The cornerstone of green hydrogen production is a well-established process known as water electrolysis. This electrochemical technique uses electricity to split water (H2O) into its fundamental components: hydrogen (H2) and oxygen (O2).
The entire reaction takes place inside a device called an electrolyzer. An easy way to think of an electrolyzer is like a battery operating in reverse. It consists of two electrodes—a positive anode and a negative cathode—separated by a conductive medium called an electrolyte.
The process unfolds in a few simple steps. Water is introduced into the electrolyzer, and a direct electrical current from a renewable source, like a dedicated solar or wind farm, is applied. This electricity provides the energy needed to break the strong chemical bonds in the water molecules, splitting them into hydrogen gas and oxygen gas.
The overall chemical equation is simple yet profound:
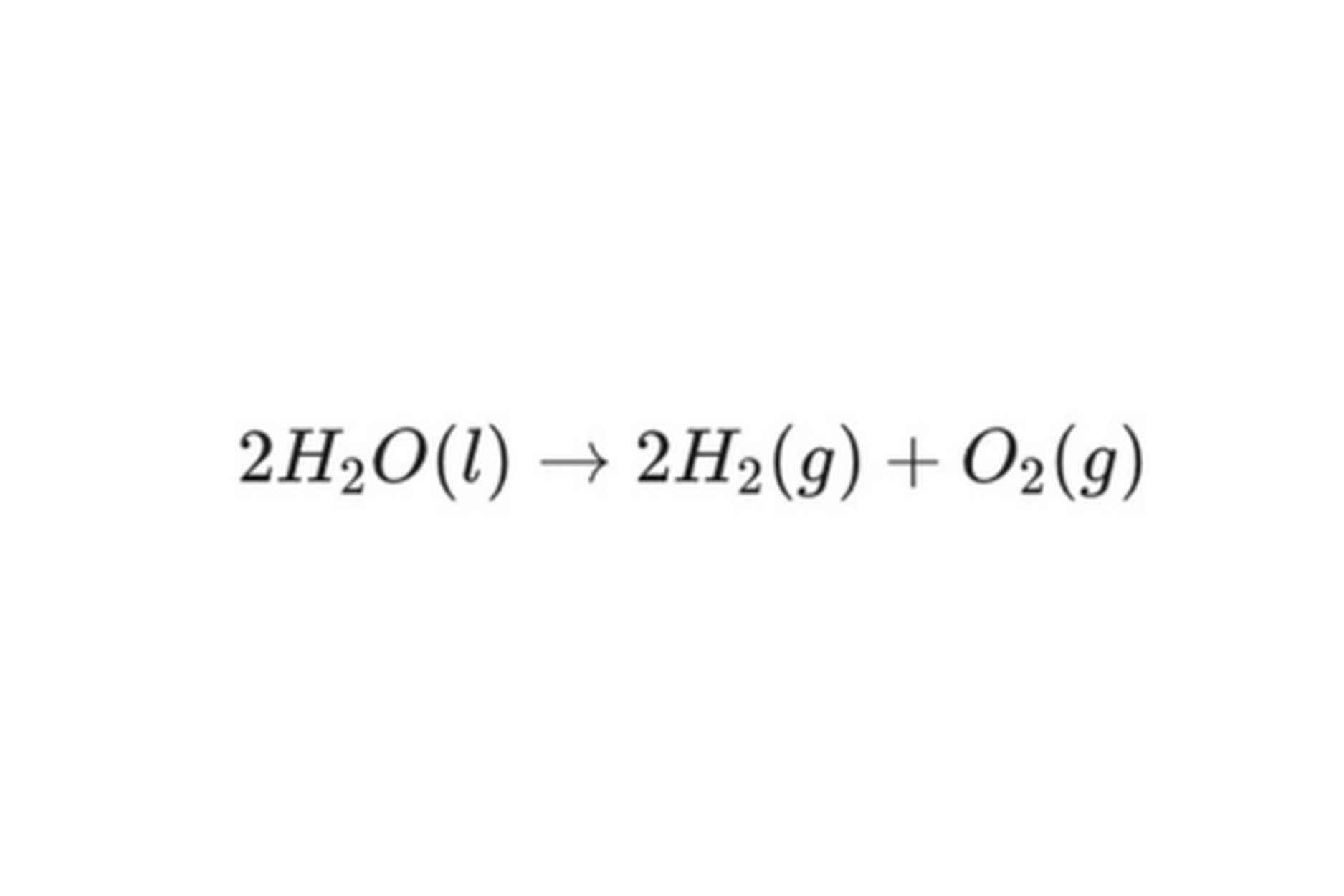
The result is high-purity hydrogen gas. For the fuel to be certified as "green," the electricity driving this entire process must be generated from 100% renewable sources.
A critical factor for economic viability is the efficiency of this process. Currently, electrolysis is at most 80% efficient, meaning a minimum of 20% of the electrical energy input is lost as heat. Producing one kilogram of hydrogen requires approximately 50-60 kWh of electricity, making energy consumption the single largest operational cost. For investors, this means that the electrical efficiency of an electrolyzer is just as important as its upfront capital cost.
The Machines That Make It Happen: A Guide to Electrolyzer Technologies
While the principle of electrolysis is the same, several distinct types of electrolyzer technologies are available. The choice of technology is a strategic decision that shapes a project's cost, performance, and ideal application.
Alkaline Electrolysis (AEL): The Established Workhorse
Alkaline electrolysis is the most mature and widely deployed technology. It uses a liquid alkaline solution as the electrolyte and relies on abundant, low-cost materials like nickel.
Advantages: Proven reliability, long operational lifespans, and the lowest capital cost.
Disadvantages: Less efficient than newer alternatives and has a slower response time, making it less ideal for pairing with the fluctuating output of solar and wind power.
Ideal Use Case: Large-scale, centralized hydrogen production facilities connected to a stable renewable power source, like a hydroelectric dam.
Proton Exchange Membrane (PEM) Electrolysis: The Flexible Innovator
PEM electrolysis is a more modern approach that uses a solid polymer membrane as the electrolyte.
Advantages: Higher energy efficiency, a compact design, and a rapid response time. This flexibility makes PEM electrolyzers perfectly suited to handle the intermittent nature of wind and solar power, allowing them to produce hydrogen when electricity is cheapest.
Disadvantages: The primary drawback is a higher capital cost due to the need for expensive precious metal catalysts like platinum and iridium.
Ideal Use Case: Projects that directly couple hydrogen production with variable renewable energy sources to capitalize on low-cost power and provide valuable grid-balancing services.
Solid Oxide Electrolyzer Cells (SOEC): The High-Efficiency Frontier
SOEC technology operates at extremely high temperatures (500-1000°C) to split steam rather than liquid water.
Advantages: The highest electrical efficiency of any electrolyzer. By using heat as part of the energy input, SOECs can be up to 26% more efficient than other types, especially when integrated with industrial processes that generate high-temperature waste heat.
Disadvantages: This is a nascent technology with challenges related to material degradation and a limited operational lifespan due to the high temperatures.
Ideal Use Case: Co-locating a hydrogen plant with an industrial facility that produces a constant stream of waste heat, such as a steel mill or ammonia plant.
Anion Exchange Membrane (AEM) Electrolysis: The Emerging Hybrid
AEM is the newest technology, aiming to combine the best attributes of AEL and PEM.
Advantages: The goal is to achieve the low material costs of AEL (using non-precious metals) while retaining the operational flexibility and compact design of PEM.
Disadvantages: As an emerging technology, AEM systems still face challenges with long-term durability and performance.
Ideal Use Case: If successful, AEM could become a disruptive, cost-effective technology suitable for a wide range of applications.
At-a-Glance Comparison of Electrolyzer Technologies
This table provides a concise overview of the key characteristics, presenting complex information in a simplified format. By making it easier to compare options side by side, it supports developers and investors in evaluating opportunities, assessing potential risks and benefits, and ultimately making well-informed decisions with greater confidence.
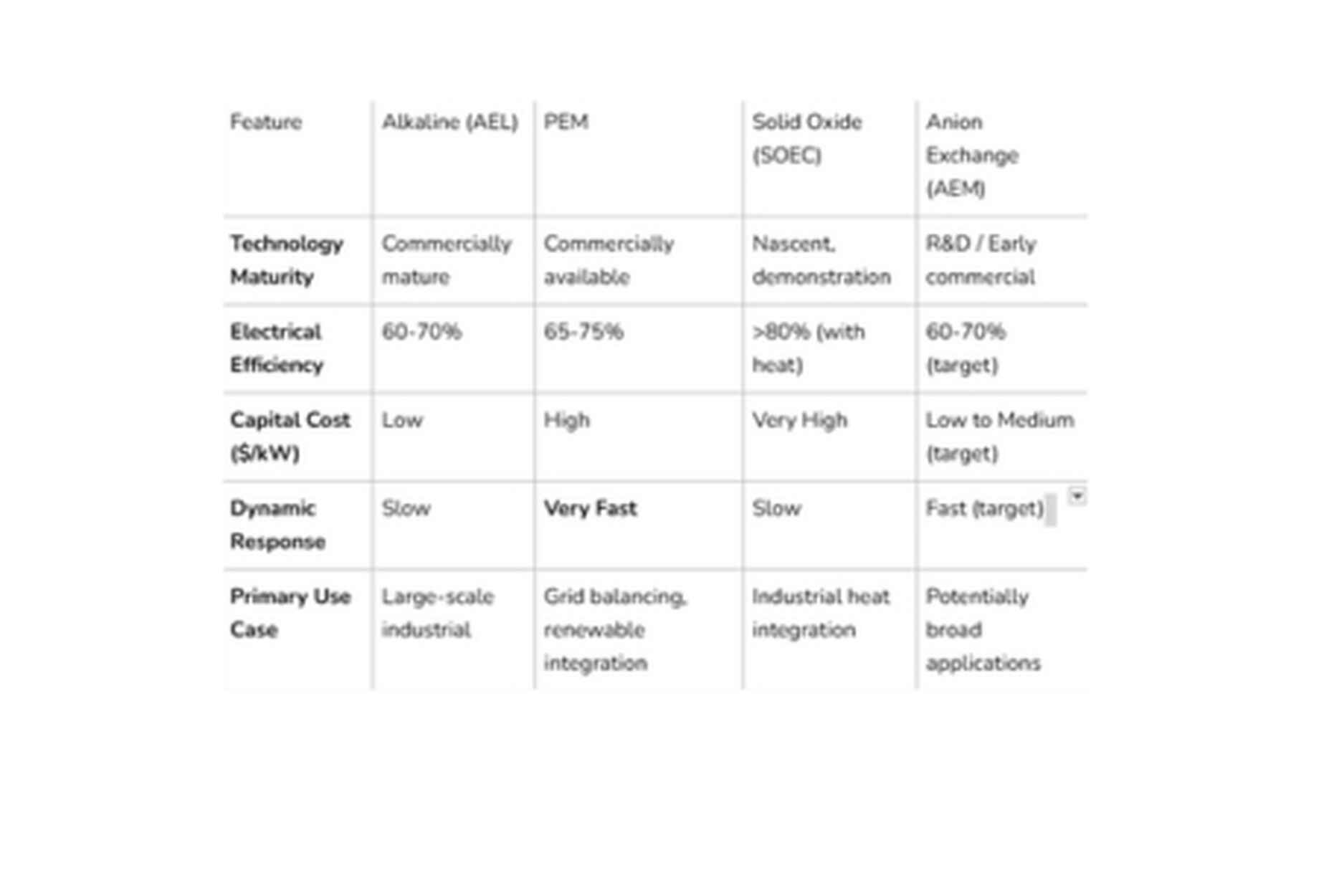
This comparison reveals a core strategic tension: the trade-off between the low cost and maturity of AEL versus the high performance and flexibility of PEM. SOEC and AEM represent distinct paths to resolve this dilemma, with SOEC focused on niche, high-efficiency applications and AEM aiming to be a "best-of-both-worlds" solution, albeit with higher technology risk.
What's Next? Future Hydrogen Production Pathways
While electrolysis is the dominant method today, researchers are actively exploring next-generation technologies that could revolutionize the field by creating a more direct, one-step process from renewable source to hydrogen. These include:
Photoelectrochemical (PEC) Water Splitting: Using semiconductor materials that absorb sunlight and split water directly, mimicking photosynthesis.
Thermochemical Water Splitting: Using high-temperature heat from concentrated solar power to drive chemical reactions that release hydrogen from water.
If commercialized, these pathways could dramatically reduce the complexity and cost of green hydrogen infrastructure, making them a critical area for long-term research and investment.
Overcoming the Hurdles on the Path to Scale
The transition to a global green hydrogen economy faces several significant hurdles that require a concerted effort to overcome.
The primary barrier is cost. Green hydrogen is currently more expensive than grey hydrogen, though global initiatives like the U.S. Department of Energy's "Hydrogen Shot" aim to drive costs down by 80% to $1 per kilogram within a decade. Other challenges include the need for a massive infrastructure build-out for storage and transport, and the sheer scale of new renewable energy required to power the electrolyzers.
This creates a classic "chicken-and-egg" problem: investors hesitate to fund production without guaranteed buyers, while industries are reluctant to convert to hydrogen until a reliable, cost-competitive supply is available. This is where strategic and stable government policy is essential to break the deadlock and create a viable ecosystem where private investment can thrive.
The VIRIDI Vision: Building the Green Hydrogen Economy Together
The journey to a decarbonized world powered by green hydrogen is both a monumental challenge and an unprecedented opportunity. The path forward is clear: electrolysis powered by renewable energy is the core technology.
At VIRIDI, we believe success requires collaboration and integration.
For Landowners: Your land is the foundation. Properties with strong solar or wind resources are prime candidates for hosting the renewable energy projects that will power our future, creating long-term, reliable revenue streams.
For Investors: Green hydrogen is a foundational long-term growth market. The most promising ventures will integrate best-in-class renewable generation with efficient hydrogen production in regions with strong policy support.
For Governments: Clear, ambitious policies are the catalyst. By focusing on developing "hydrogen hubs" and creating demand, governments can unlock the full economic and environmental potential of green hydrogen.
The future of energy is about building interconnected systems. We invite you to partner with us to build a cleaner, more resilient, and prosperous energy future.
